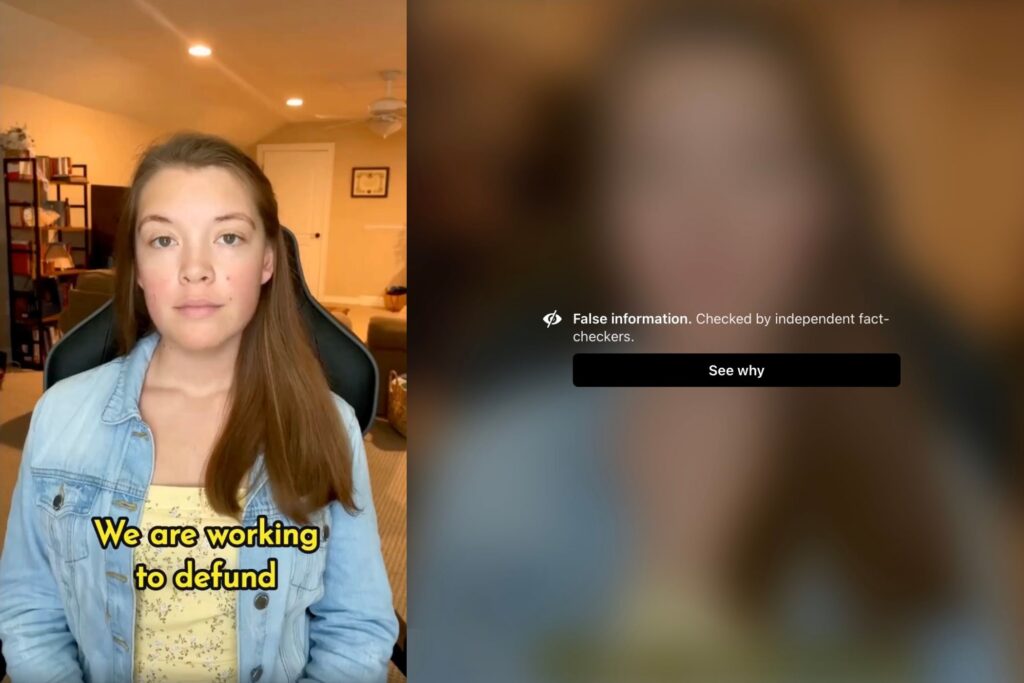
Chiara McKenna
04/15/24

Bishop Fulton Sheen and Chairman Mao Agreed: Hate Drives Communism
Steven W. Mosher
April 8, 2024

In a Shock to Their Government, the Irish Defend the Family
Carlos Beltramo, Ph.D. | PRI European Office
April 1, 2024

Response to Archbishop John Wester
PRI Staff
March 26, 2024
Facebook Censors PRI’s Petition to Stop the Pandemic Treaty and Defund the WHO
Chiara McKenna
April 15, 2024

Bishop Fulton Sheen and Chairman Mao Agreed: Hate Drives Communism
Steven W. Mosher
April 8, 2024

In a Shock to Their Government, the Irish Defend the Family
Carlos Beltramo, Ph.D. | PRI European Office
April 1, 2024

Response to Archbishop John Wester
PRI Staff
March 26, 2024

Catholic Relief Services Programs Present 5 Expressions of Grave Evildoing
Chiara McKenna
March 19, 2024

PRI Investigation Finds CRS In Violation Of Papal Teaching
Chris Manion, PhD
March 12, 2024

Yes, COVID came from a lab. When will the mainstream media quit gaslighting us?
Steven W. Mosher
March 4, 2024

Them versus Us
Steven W. Mosher
February 26, 2024

Catholic Relief Services Programs Present 5 Expressions of Grave Evildoing
Chiara McKenna
March 19, 2024

PRI Investigation Finds CRS In Violation Of Papal Teaching
Chris Manion, PhD
March 12, 2024

Yes, COVID came from a lab. When will the mainstream media quit gaslighting us?
Steven W. Mosher
March 4, 2024

Them versus Us
Steven W. Mosher
February 26, 2024

Abortion by Mail
Samantha Harris
February 20, 2024

The Globalists Have a New Goal:
Steven W. Mosher
February 12, 2024
Never miss an update!
Get our Weekly Briefing! We send out a well-researched, in-depth article on a variety of topics once a week, to large and growing English-speaking and Spanish-speaking audiences.